Table of Contents
As healthcare settings increasingly rely on point-of-care diagnostics, the use of Chlamydia Rapid Test Kits – has grown substantially. These kits are designed to offer rapid, accessible testing, an infection that continues to be among the most commonly reported sexually transmitted infections worldwide. However, with the increase in diagnostic convenience comes a critical need for structured protocols surrounding the safe handling and disposal of these medical devices. Improper disposal or mishandling of Chlamydia Test Kits can lead to biohazard exposure, environmental contamination and non-compliance with health regulations.
The integrity of healthcare environments and safety of medical staff and patients depends on robust guidelines that address the full lifecycle of these kits – from storage and handling to post-use decontamination and final disposal. This article outlines technically-sound, regulation-compliant practices for managing Chlamydia Screening Kits in clinical and laboratory environments.
Understanding the Risks of Handling Chlamydia Test Kits
The biological nature of Chlamydia Detection through in vitro diagnostics presents inherent biohazard risks. Specimens collected during Chlamydia Swab Tests processes contain bodily fluids potentially harboring infectious agents. Even though Chlamydia Home Kits and clinical test kits are typically designed for single-use, any contact with the specimen material – whether through accidental spills, incorrect handling or improper sealing – can pose risks to healthcare professionals.
Additionally, components such as reaction buffers, test cassettes, pipettes and collection swabs may retain traces of the specimen or chemicals that require careful disposal. These are not neutral materials; they must be considered contaminated post-use and managed accordingly to prevent cross-contamination or accidental pathogen transmission. Facilities must classify used Rapid Chlamydia Test components under biohazardous waste, with clear boundaries between clean and contaminated zones to mitigate these risks.

Designated Testing and Disposal Zones
Healthcare facilities that use test kitsin supervised settings or operate clinical diagnostics with Chlamydia Test Strips must designate specific areas for test administration and post-test disposal. This separation minimizes the risk of environmental contamination and ensures that potentially infectious materials are contained. Testing areas must be clearly labeled and only accessible to trained personnel, while disposal zones must include appropriate containment equipment such as biohazard bins, sharps containers and leak-proof disposal bags.
Facilities should integrate these areas into their infection control architecture, ensuring spatial and procedural separation between patient contact areas, sterile supply storage and biohazard disposal points. Implementing physical barriers and visual signage further enhances the efficacy of containment protocols, ensuring that Chlamydia Test Kit components are managed in a controlled and compliant manner.
Personal Protective Equipment and Hygiene Protocols
The handling of diagnostic kits, especially those involving biological samples, requires strict adherence to personal protective equipment (PPE) protocols. When managing home kits, staff must wear gloves, lab coats, face masks and protective eyewear to shield against splash exposure and contact with infectious materials.
Gloves must be changed between each patient interaction and disposed of as biohazard waste. Additionally, hand hygiene remains paramount – personnel should sanitize or wash hands thoroughly before donning gloves and immediately after glove removal. PPE should be stored in a clean environment and replaced immediately if damaged or contaminated during the testing process. The goal is not only to protect the individual but also to uphold sterile conditions around the Chlamydia Detection process, reducing the likelihood of erroneous results or test kit compromise.
Procedures for Specimen Collection and Kit Usage
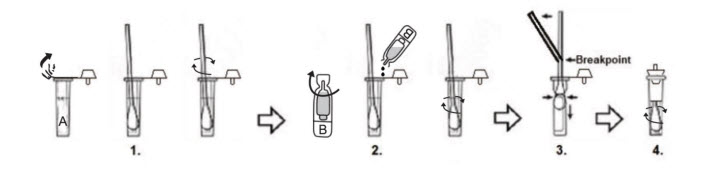
Proper specimen collection plays a central role in both the accuracy of Chlamydia Test Kits and the safety of their use. For clinical applications, tests must be collected using aseptic techniques, ensuring that swabs are not contaminated prior to sample acquisition. Once collected, the specimen must be immediately inserted into the buffer solution or processing cassette provided with the test kit. Any deviation from this protocol – such as delayed insertion, contamination of swab tips or improper sealing – can compromise the test and increase exposure risk.
Technicians must strictly follow manufacturer instructions, including using each kit within its expiration window and under controlled environmental conditions. Kits should remain sealed until use and all components – such as pipettes, buffer tubes and reaction cassettes – must be disposed of immediately after a single use. Reuse or partial use is not permitted under any regulatory standard and may lead to false readings and biohazard incidents. Understanding that Chlamydia Rapid Test Kit components are designed for one-time use is essential to maintaining both diagnostic integrity and staff safety.
Packaging and Containment of Used Kits
After the completion of each Chlamydia Rapid Test, all used materials must be treated as biohazard waste. This includes the swab, any remaining buffer solution, test cassette, gloves and any paper towel or surface protectors that may have come into contact with the specimen. These items should be placed directly into a certified biohazard bag or container – ideally one that is puncture-resistant, leak-proof and sealable.
Facilities must ensure that these containers are emptied and processed according to state or national biohazard disposal guidelines. Waste logs should be maintained to document the volume, disposal method and responsible personnel for each batch of waste. When disposing of materials from Chlamydia Screening Kits, the focus must be on preventing leakage, avoiding manual handling of contents once sealed and ensuring transport to an authorized medical waste processor.
Any surface or workbench exposed to the specimen or kit components must be thoroughly disinfected using hospital-grade agents with proven efficacy against enveloped viruses and bacterial pathogens. Cleaning protocols should be detailed in the facility’s standard operating procedures (SOPs) and followed without exception.
Chemical Reagent Handling and Disposal
Many Chlamydia Test Kits, particularly rapid formats used in both clinical and home settings, contain chemical reagents essential for the detection of antigens or antibodies. These reagents may include buffers, preservatives and chromogenic substances. Although typically present in small quantities, these substances can pose health and environmental risks if mishandled. For instance, some reagents may be skin irritants, while others could pose inhalation hazards or environmental toxicity.
Healthcare providers must ensure that any residual liquids or reagent vials are handled in accordance with their corresponding Material Safety Data Sheets (MSDS). If a kit component includes a reagent classified as hazardous, it must be disposed of separately from general biohazardous waste and treated as chemical waste. Designated chemical waste containers should be available in all facilities where Chlamydia Detection testing is performed. Staff must be trained to recognize these components and understand the appropriate disposal workflows.
Moreover, facilities must never mix chemical waste with biological materials unless permitted under local regulations. Cross-contamination can lead to complex disposal challenges and potential safety hazards. Where permitted, incineration may be used for combined disposal, but autoclaving is usually reserved for biological content alone.
Storage Requirements and Expiry Protocols
Correct storage of Chlamydia Rapid Test Kits is critical not just for test performance but also for safe handling. Kits must be kept within the temperature and humidity ranges specified by the manufacturer. This typically involves storing them in cool, dry environments away from direct sunlight and chemical agents. Most kits, including those used in Chlamydia Home Tests, contain heat-sensitive materials that can degrade under improper conditions.
Healthcare facilities must implement inventory management practices such as first-in-first-out (FIFO) to ensure that no expired kits are inadvertently used. Each test should be visually inspected prior to use to confirm the integrity of its seal, absence of damage and readability of expiration dates. Expired or compromised kits must be removed from inventory immediately and disposed of in compliance with hazardous or biohazardous waste protocols.
Tracking expiration dates not only ensures test accuracy but also minimizes liability in case of adverse incidents or test failures. Documentation of expired test disposal should be maintained, including lot numbers, quantities and disposal methods. This reinforces traceability and enhances regulatory compliance during inspections or audits.

Staff Competency and Continuous Training
Safe handling and disposal of Chlamydia Test Kits depend heavily on the knowledge and vigilance of the personnel administering the tests. It is not sufficient to distribute written protocols; ongoing competency training must be part of a facility’s operational culture. All staff involved in Chlamydia Screening Kit use, from sample collectors to laboratory technicians, must undergo structured training that covers PPE usage, test administration, contamination control and waste handling.
Training modules should be updated periodically to reflect changes in regulatory guidelines, manufacturer updates or internal audits. Annual refresher courses ensure that knowledge remains current, especially in high-turnover environments. Facilities may also conduct random spot-checks or competency assessments to identify gaps in adherence to safety protocols.
Documentation of training sessions, participant sign-offs and competency evaluations should be preserved as part of institutional records. In the event of an exposure incident or quality review, these records provide critical evidence of due diligence and commitment to best practices in managing Chlamydia Home Kit and clinical diagnostic systems.
Conclusion
The use of Chlamydia Rapid Test Kits and related diagnostic tools is indispensable in modern healthcare settings, offering quick, reliable detection of a prevalent and often asymptomatic infection. However, these benefits can only be realized when the test kits are handled and disposed of with clinical precision and regulatory awareness. From initial specimen collection through post-use decontamination, each stage must adhere to well-defined protocols that prioritize safety, integrity and compliance.
Healthcare facilities must initialize best practices covering everything from PPE and reagent management to expired kit disposal and spill response. By doing so, they not only reduce biohazard exposure and environmental risks but also enhance diagnostic reliability and operational efficiency. With structured training, proper infrastructure and vigilant oversight, professionals can safely harness the advantages of Chlamydia Screening Kits while maintaining the highest standards of infection control and workplace safety.
FAQs
What should be done with used Chlamydia Swab Tests?
Used swabs and test parts should be placed directly into biohazard containers. These materials are potentially infectious and need to be treated through autoclaving or incineration by approved medical waste services.
Are Chlamydia Home Test Kits safe to dispose of at home?
No. Even though these kits are used at home, they still contain biological materials. They should be taken to medical waste drop-off points or disposed of according to local health authority guidelines.
How can healthcare workers prevent contamination when using Chlamydia Test Strips?
By wearing proper protective gear like gloves and masks, following sterile techniques and disposing of all used materials immediately in designated biohazard containers.
What is the correct way to dispose of expired Chlamydia Test Kits?
Expired kits should not be used. They must be treated as biohazardous waste and disposed of through approved processes like incineration or autoclaving.
Is training required for staff using Chlamydia Detection Kits?
Yes. All staff must be trained on how to safely handle, use and dispose of the kits to ensure safety and follow legal requirements.

