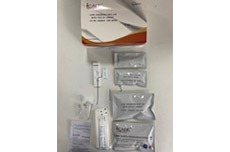
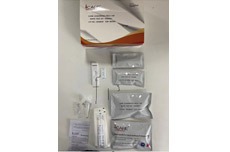
STD Rapid Test Kit (HIV 1&2 Oral Swab, Chlamydia, Hepatitis Test)
Disclaimer: This assay provides preliminary analytical test results. A definitive conclusion should only be made by a qualified physician based on further relevant clinical and laboratory findings and evaluations.
We manufacture an extensive range of home use, private-use STD tests and professional use in hospitals.
Our current range of STD home tests includes:
- iCARE HIV 1&2 rapid field test
- iCARE Gonorrhoea Field test
- iCARE Syphilis Field test
- iCARE Chlamydia Field test
- iCARE Hepatitis B Field test
- iCARE Hepatitis C Field test
- iCARE HIV 1&2 ORAL Swab Field test
- iCARE Herpes2 rapid Field test
- OTC boxes are sold separately.
- iCare Advanced HIV 1&2 rapid field test
- iCare Advanced Gonorrhea rapid field test
- iCare Advanced Syphilis rapid field test
- iCare Advanced Chlamydia Field test
- iCare Advanced iCARE Hepatitis B rapid field test
- iCare Advanced Hepatitis C rapid field test
- iCare Advanced Herpes II rapid field test
- NEW PRODUCT: iCare Advanced HIV 1&2 Home Use Oral Swab Rapid Test Kit
- NEW PRODUCT: iCare HIV 1&2 4th GEN HIV 1&2 Home Test Kit
- Ok Vita Meter | Icare Vita Glucose Monitoring System
For more information on SG Diagnostics products & related services, please visit our website by scanning this QR code.

iCare Advanced HIV 1&2 Oral Swab rapid test
The iCARE HIV 1&2 Rapid Screen Test is an exceptional option for detecting undiagnosed HIV infections with high precision, speed, and affordability. These HIV 1&2 home test kits offer numerous advantages, including ease of use, non-invasive sample collection, cultural acceptance, high accuracy, and quick results. Compared to blood-based testing methods, these fast HIV 1&2 home use test kits provide significant benefits. The iCARE HIV 1&2 Rapid Screen Test demonstrates a high specificity and sensitivity in detecting HIV antibodies in oral fluid samples, surpassing traditional EIAs. These test kits are readily available in bulk supply.
With HIV 1&2 home use test kits, you can obtain results in just a few minutes, enabling prompt initiation of treatment and reducing the risk of transmitting the virus to others. The convenience and speed of these HIV 1&2 home test kits contribute to early diagnosis and improved management of HIV. Additionally, the accessibility and privacy of using these test kits in the comfort of your own home make them an appealing option for many individuals seeking regular HIV screening.
iCARE HBsAg (Hepatitis B Surface Antigen) Rapid Test Kit
Hepatitis B Core Antibodies are qualitatively detected in human blood or plasma samples using the Hepatitis B Surface Antigen Rapid Screen Test. This Hepatitis B Rapid Screen Test gives results within few minutes and is cost-effective and simple to use.
iCARE HIV 1&2 Rapid Test Kit (Whole Blood/Serum/Plasma)
HIV infection can be discovered with the HIV Plasma Rapid Screen Test. The results of certain tests are not available for many days, but results from the HIV 1&2 Rapid Screen Test can be obtained in about 15 minutes. This HIV 1&2 Whole Blood Rapid Screen Test helps medical professionals to identify HIV infection early, enabling patients to seek treatment more quickly.
iCARE Syphilis Rapid Screen Test
The Syphilis Rapid Screen Test, which tests people at home using a little blood, serum, or plasma sample, makes it simple to identify syphilis induced by the spirochete bacteria. Results are available immediately and no laboratory work is necessary.
iCARE HBsAb (Hepatitis B Surface Antibody) Rapid Screen Test
Using a blood sample, the hepatitis B surface antigen may be quickly and accurately detected using the Hepatitis B Surface Antibody Rapid Screen Test. This test is a straightforward, quick, and simple-to-interpret method for determining whether or not you have hepatitis B. The outcome is interpreted in terms of coloured lines that appear or do not in the test region.
STD Rapid Test Kit – iCare Advanced Chlamydia Field Test
The iCARE CHLAMYDIA FIELD USE RAPID TEST KIT KIT is a rapid home test kit specifically designed for the direct detection of Chlamydia. This product is intended for in vitro diagnostic use only and can be conveniently used at home. Chlamydia is a curable sexually transmitted infectious disease (STD) caused by the bacteria Chlamydia trachomatis. With our Chlamydia Rapid home test kit, you can discreetly detect Chlamydia in the privacy of your own home. Our test kit is user-friendly, and the results can be interpreted within just 15 minutes.
Chlamydia home test kits like the iCARE CHLAMYDIA FIELD USE RAPID TEST KIT offer a convenient and confidential means of detecting Chlamydia infection. By providing individuals with the ability to test for Chlamydia at home, these test kits promote early detection, timely treatment, and prevention of further transmission. It is important to remember that accurate and timely diagnosis, followed by appropriate medical intervention, is crucial in managing Chlamydia infections effectively.
By using Chlamydia home test kits, individuals can take control of their sexual health and seek appropriate medical care if the test results indicate a positive Chlamydia infection. Regular testing and early treatment are essential in preventing complications associated with Chlamydia and safeguarding personal and public health.
Do send us your inquiry so as to allow us to address your specific requirements.
ICARE ADVANCED HSV-2 IgG FIELD USE RAPID TEST KIT
iCARE HSV-2 IgG Field Use Rapid Test Kit is a rapid test based on the principle of immunoassays combined with conjugated colloid gold technology. This diagnostic device is a test for qualitative detection of anti-HSV-2 IgG in human whole blood, serum or plasma.
Herpes is among several inflammatory viral diseases of the skin or nervous system, especially herpes simplex, characterized by the formation of small watery blisters. There are two kinds of Herpes, Type 1 and Type 2. Herpes Simplex Type 1 typically involves an infection of the mouth with what are commonly known as cold sores or fever blisters and is called oral herpes. Herpes Simplex Type 2, called genital herpes, is a sexually transmitted disease (STD) that usually affects the genital area with very similar blisters as is seen with Type 1. The herpes simplex virus can cause an individual to suffer from blisters or cold sores. It is also possible to have contracted genital herpes and to suffer no symptoms at all or to be asymptomatic. Transmission of herpes simplex most commonly occurs by direct contact with a blister or the body fluids, including saliva, semen, vaginal fluid or the fluid from herpetic blisters of another infected person. Herpes simplex virus (HSV)-2 is periodically shed in the human genital tract, most often asymptomatically, and most sexual transmissions occur during asymptomatic shedding.
ICARE ADVANCED HIV 1&2 4th GEN HOME TEST KIT
iCare Advanced HIV 1&2 4th GEN HIV 1&2 Home Test Kit is a tri-line, serological, lateral flow chromatographic immunoassays for the simultaneous and qualitative detection of human immunodeficiency virus type 1 (HIV-1) p24 antigen, human immunodeficiency virus type 1 antibody and type 2 antibody in human fingertip whole blood specimens to aid in the diagnosis of infection with HIV. The test only provides preliminary analysis results but not critical diagnosis criteria. Any reactive specimen with the iCare Advanced HIV 1&2 4th GEN HIV 1&2 Home Test Kit must be analyzed and confirmed with alternative testing method(s) and clinical findings. The test is intended for healthcare professional use, self-test, or home test for preliminary screening of HIV infection by layperson.
ICARE ADVANCED ANTI-HEPATITIS C RAPID FIELD TEST
iCARE Advanced Anti-Hepatitis C Rapid Field Test is a colloidal gold enhanced, rapid immunochromatographic assay for the qualitative detection of antibodies to Hepatitis C Virus (HCV) in human whole blood, serum or plasma. This test is a screening test and all positives must be confirmed using an alternate test such as western blot. The test is intended for field and professional use only.
The assay starts with a sample applied to the sample well and provided diluent added immediately. The HCV antigen colloidal gold conjugate embedded in the sample pad reacts with the HCV antibody present in blood; serum or plasma forming conjugate/HCV antibody complex. As the mixture migrates along the test strip, the conjugate/HCV antibody complex is captured by a recombinant HCV antigen which contains core, NS3, NS4, NS5 immobilized on a membrane forming a colored test band in the test region. A negative sample does not produce a test line due to the absence of colloidal gold conjugate/HCV antibody complex. The antigens used in the test are recombinant proteins corresponding to highly immunoreactive regions of HCV. A colored control band in the control region appears at the end of the test procedure regardless of the test result.
ICARE ADVANCED GONORRHEA HOME USE RAPID TEST KIT
iCARE Advanced Gonorrhea Home Use Rapid Test Kit (Swab) is a rapid home test kit for direct detection of Gonorrhea. Gonorrhea is a curable sexually transmitted infectious disease (STD), which is caused by the bacterium Neisseria gonorrhoeae. ICARE Advanced Gonorrhea Home Use Rapid Test Kit (Swab) offers a convenient and confidential means of detecting Gonorrhea infection. By providing individuals with the ability to test for Gonorrhea at home, these test kits promote early detection, timely treatment, and prevention of further transmission. It is important to remember that accurate and timely diagnosis, followed by appropriate medical intervention, is crucial in managing Gonorrhea infections effectively.
By using Gonorrhea home test kits, individuals can take control of their sexual health and seek appropriate medical care if the test results indicate a positive Gonorrhea infection. With our Gonorrhea Rapid home test kit, you can detect Gonorrhea on your own in 100% privacy. This product is for in vitro diagnostic use only. The results can be interpreted within just 15 minutes.
STD RAPID TEST KIT – ICARE ADVANCED PF/PV HOME USE RAPID TEST KIT
iCARE Advanced Malaria P.f/P.v Home Use Rapid Test Kit (Blood) is a rapid self-performing, qualitative, two sites sandwich immunoassays, utilizing whole blood for the detection of P.falciparum specific histidine rich protein-2 (P.f HRP-2) and P.vivax specific pLDH. The test may also be used for specific detection and differentiation of P.falciparum and P.vivax malaria in whole blood samples and for follow up of anti-malarial therapy. This kit is for in vitro diagnostic use only and is for both healthcare professional use and field use.
ICARE ADVANCED HIV/SYPHILIS TRI-LINE FIELD TEST KIT
iCARE HIV/Syphilis Tri-line Field Test Kit is a tri-line, serological, lateral flow chromatographic immunoassays for the qualitative detection of human immunodeficiency virus (HIV) type 1 antibody, type 2 antibody, and antibodies (IgG and IgM) to Treponema Pallidum in human whole blood, serum, or plasma specimens to aid in the diagnosis of infection with HIV and/or Syphilis. The test only provides preliminary analysis results. Any reactive specimen with the iCARE HIV/Syphilis Tri-line Field Test Kit must be analyzed and confirmed with alternative testing method(s) and clinical findings. The test is intended for healthcare professional use and field use. With our HIV/Syphilis Tri-line Field Test Kit, you can detect HIV/Syphilis on your own in 100% privacy. This product is for in vitro diagnostic use only. The results can be interpreted within just 15 minutes. Applications of the test including, screening test for sex transmitted diseases among high-risk group of people, regular health examinations, and field screen test for blood bank.
FAQs About STD Rapid Test Kit
STD Rapid Screen Test (HIV 1&2 Oral Swab, Chlamydia, Hepatitis Test)
1. How does the iCare Advanced HIV 1&2 Oral Swab Rapid Test Kit contribute to the global fight against HIV/AIDS?
<p align="justify">The iCare Advanced HIV 1&2 Oral Swab Rapid Test Kit goes beyond regional customization and actively contributes to the global efforts to combat HIV/AIDS.</p>
<ul>
<li>WHO approval: The kit is endorsed by the World Health Organization (WHO), attesting to its quality and reliability. This global recognition makes it a trusted tool in the hands of healthcare professionals worldwide.</li>
<li>Research and development: Continuous research and development efforts ensure that the kit stays abreast of advancements in HIV testing technology. Regular updates and improvements contribute to its efficacy in detecting HIV infections accurately.</li>
<li>Accessibility initiatives: The manufacturers of the iCare Advanced HIV 1&2 Oral Swab Rapid Test Kit actively participate in global accessibility initiatives, ensuring that the kit reaches even the most underserved populations. This commitment aligns with the broader goal of achieving universal access to HIV testing and treatment.</li>
</ul>
2. How is the iCare Advanced HIV 1&2 Oral Swab Rapid Test Kit tailored for African countries?
<p align="justify">The iCare Advanced HIV 1&2 Oral Swab Rapid Test Kit is specifically designed for the unique healthcare challenges in African countries. It takes into consideration the prevalent conditions and healthcare infrastructure in these regions.</p>
<ul>
<li>It requires minimal training: The test kit is user-friendly and does not necessitate extensive training. This is crucial in regions where healthcare resources are limited, ensuring that even personnel with basic training can administer the test effectively.</li>
<li>Temperature resilience: The kit is designed to withstand varying temperature conditions, a critical feature for African countries with diverse climates. This ensures the reliability of results even in areas where temperature control may be challenging.</li>
<li>Cost-effectiveness: Recognizing the budget constraints faced by many healthcare systems in African countries, the iCare Advanced HIV 1&2 Oral Swab Rapid Test Kit is an affordable solution, making HIV testing more accessible to a broader population.</li>
</ul>
3. How is the iCare HBsAg Rapid Screen Test suitable for diverse populations in Indo China?
<p align="justify">Recognizing the diverse populations in Indo China, the iCare HBsAg Rapid Screen Test is engineered to accommodate the unique healthcare needs of various ethnic groups and communities.</p>
<ul>
<li>Validation with Diverse Population Groups: The test has undergone rigorous validation studies, including diverse population groups found in Indo China. This ensures its accuracy and reliability across different ethnicities.</li>
<li>Inclusivity in Design: The packaging, instructions, and overall design of the test kit are created with inclusivity in mind. This approach ensures that individuals from various backgrounds can easily understand and use the test effectively.</li>
<li>Adherence to Cultural Practices: The iCare HBsAg Rapid Screen Test respects cultural practices prevalent in Indo China, promoting higher acceptance and participation in Hepatitis B screening initiatives.</li>
</ul>
4. How does the iCare HBsAg Rapid Screen Test address the Hepatitis B scenario in Indo China?
<p align="justify">The iCare HBsAg Rapid Screen Test is specifically designed to tackle the Hepatitis B situation in Indo China, considering the prevalence of the virus and the regional healthcare infrastructure.</p>
<ul>
<li>High Sensitivity and Specificity: The test exhibits high sensitivity and specificity, crucial for accurate detection of Hepatitis B surface antigen. This reliability is particularly important in Indo China, where accurate diagnosis plays a pivotal role in disease management.</li>
<li>Rapid Results: The test provides quick results, enabling healthcare professionals to initiate timely interventions and necessary follow-up actions. This feature aligns with the urgency often required in managing Hepatitis B cases in Indo China.</li>
<li>Affordable Solution: Recognizing the economic constraints in the region, the iCare HBsAg Rapid Screen Test offers an affordable yet high-quality solution for Hepatitis B screening, making it accessible to a broader population.</li>
</ul>
5. How does the iCARE Syphilis Rapid Screen Test cater to the healthcare infrastructure diversity in Central Asia?
<p align="justify">Central Asia exhibits diversity in healthcare infrastructure among the listed countries. The iCARE Syphilis Rapid Screen Test acknowledges this variation and adapts to ensure seamless integration into different healthcare systems.</p>
<ul>
<li>iCARE provides customizable implementation plans, considering the unique healthcare setups of Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, and Uzbekistan.</li>
<li>Collaboration with local health authorities is prioritized to align the iCARE test with existing healthcare practices in each country.</li>
<li>Flexibility in deployment enables the iCARE test to be effectively utilized in both well-established healthcare facilities and more remote areas.</li>
</ul>
6. What is the significance of syphilis in Central Asia, specifically in the listed countries?
<p align="justify">Syphilis holds significance in Central Asia, encompassing Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, and Uzbekistan, due to various factors such as regional healthcare dynamics, cultural practices, and the prevalence of the infection.</p>
<ul>
<li>The prevalence of syphilis varies across these countries, necessitating region-specific approaches for effective control.</li>
<li>Cultural factors influence healthcare-seeking behaviors, requiring tailored strategies to address syphilis awareness and prevention.</li>
<li>Collaborative efforts among Central Asian nations are essential to combat syphilis collectively and share best practices.</li>
</ul>
7. What is the iCARE Chlamydia and Gonorrhea Combo Test Kit and how it is useful to Caribbean Individuals?
The iCARE Chlamydia and Gonorrhea Combo Test Kit is a reliable at-home testing solution designed to detect the presence of Chlamydia and Gonorrhea infections. This test kit allows individuals in Caribbean countries, including Anguilla, Antigua and Barbuda, Aruba, the Bahamas, Barbados, Belize, Bermuda, British Virgin Islands, Cayman Islands, Cuba, Dominica, Dominican Republic, Grenada, Guyana, Jamaica, Haiti, Montserrat, Netherlands Antilles, and Puerto Rico, to conveniently and confidentially test for these sexually transmitted infections (STIs) without visiting a healthcare facility.