Table of Contents
Co-Branded Hepatitis C Rapid Tests
Co-branded Hepatitis C rapid tests play a crucial role in improving the accuracy and accessibility of diagnostic tools for Hepatitis C. These tests are developed in collaboration between multiple organizations, which enhances their credibility and effectiveness. WHO PQ Listed Co-Branded Hepatitis C Rapid Test, WHO PQ Listed HCV Rapid Test, and WHO PQ Listed Hep C Rapid Test ensures adherence to stringent international standards, making it a reliable choice for accurate diagnosis.
The benefits of co-branding extend beyond just quality assurance. It also facilitates broader distribution and accessibility, particularly in underserved regions. By partnering with established organizations, manufacturers like Jal Medical can leverage their expertise to deliver high-quality diagnostic solutions globally. This collaboration not only enhances the test’s reliability but also supports global health initiatives aimed at combating Hepatitis C.
The Role of Co-Branding in Rapid Tests
Co-branding in rapid tests involves the partnership between manufacturers and other reputable organizations to produce and market diagnostic tools. This strategy combines expertise and resources, leading to improved test performance and increased trust among healthcare providers. The WHO PQ Listed Co-Branded Hepatitis C Rapid Test exemplifies this approach by meeting rigorous international standards, ensuring accuracy and reliability.
The co-branding process also helps in establishing the credibility of the tests in various markets. By associating with trusted brands, the tests gain broader acceptance and support from global health organizations. This enhanced credibility is crucial for manufacturers like Jal Medical, as it facilitates the adoption of their diagnostic solutions worldwide and contributes significantly to effective Hepatitis C control efforts.
Jal Medical’s Role and Contribution
Jal Medical is a leading manufacturer, supplier, and exporter of diagnostic tools, including the WHO PQ Listed Hepatitis C Rapid Test. Our commitment to quality and innovation ensures that their tests meet the highest international standards.
As a recognized player in the healthcare industry, Jal Medical’s contribution to WHO PQ Listed Co-Branded Hepatitis C Rapid Tests is significant in advancing global health initiatives.
By focusing on rigorous testing and adherence to WHO PQ standards, Jal Medical enhances the reliability and effectiveness of their rapid tests. This dedication supports efforts to improve Hepatitis C diagnosis and treatment worldwide. Our role in producing high-quality, co-branded tests underscore their commitment to improving healthcare outcomes and facilitating global access to essential diagnostic tools.
Importance of WHO PQ Listing for Hepatitis C Rapid Tests
The WHO PQ Listing is essential for Hepatitis C rapid tests because it signifies adherence to international quality standards. This listing process ensures that the tests are thoroughly evaluated for accuracy, reliability, and performance before they are approved for use. The WHO PQ Listed Co-Branded Hepatitis C Rapid Test meets these rigorous criteria, providing healthcare professionals with a trusted tool for diagnosing Hepatitis C.
Furthermore, the WHO PQ Listing facilitates the global distribution of these tests, making them accessible in resource-limited settings where they are most needed. By supporting global health programs and initiatives, these tests play a pivotal role in enhancing the effectiveness of Hepatitis C control efforts and improving health outcomes worldwide.
The Benefits of Co-Branded Hepatitis C Rapid Tests
Co-branded Hepatitis C rapid tests offer several advantages, including enhanced accuracy and broader market acceptance. These tests benefit from the combined expertise of multiple organizations, which results in improved diagnostic performance and increased trust among healthcare providers. The WHO PQ Listed Co-Branded Hepatitis C Rapid Test is a prime example of this synergy, meeting high international standards.
Additionally, co-branding helps in expanding the reach of these tests by leveraging the networks and reputations of partnering organizations. This approach not only boosts the test’s credibility but also supports its distribution in various regions. For manufacturers like Jal Medical, this collaboration is instrumental in ensuring that high-quality tests make a significant impact on global Hepatitis C control efforts.
Ensuring High Quality and Accuracy
The quality and accuracy of Hepatitis C rapid tests are paramount for effective diagnosis and treatment. WHO PQ Listed Hepatitis C Rapid Tests are subjected to rigorous evaluation processes to ensure they meet the highest standards of performance. This listing process involves thorough testing for accuracy, reliability, and consistency, ensuring that the tests provide correct results and are dependable for clinical use.
By adhering to these stringent standards, the WHO PQ Listed Co-Branded Hepatitis C Rapid Test provides healthcare providers with a reliable tool for early detection. Accurate results are crucial for initiating timely treatment and managing Hepatitis C effectively, ultimately improving patient outcomes and supporting global health initiatives.
Facilitating Global Distribution and Access
WHO PQ Listing plays a critical role in facilitating the global distribution of Hepatitis C rapid tests. This listing helps in navigating regulatory requirements and ensures that the tests are recognized and accepted in various countries. The WHO PQ Listed Co-Branded Hepatitis C Rapid Test benefits from this global recognition, making it easier to distribute in different regions.
Moreover, the listing supports efforts to increase access to these tests in low-resource settings. By meeting international standards, these tests can be introduced into healthcare systems in underserved areas, helping to bridge the gap in diagnostic capabilities and support global Hepatitis C control programs. This enhanced accessibility is vital for achieving widespread and effective disease management.
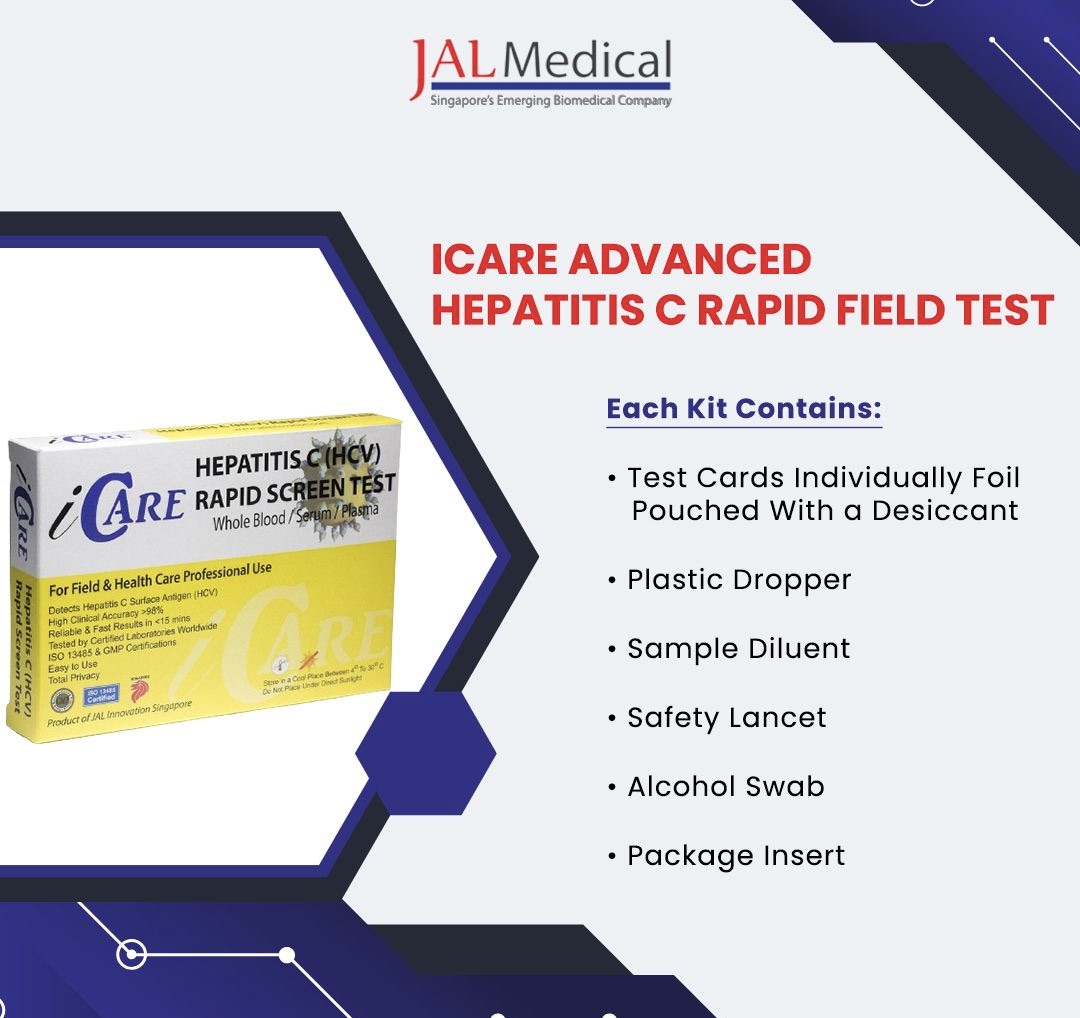
Supporting Global Hepatitis C Control Efforts
The WHO PQ Listed Hepatitis C Rapid Test plays a pivotal role in supporting global Hepatitis C control efforts. By providing a reliable means of early detection, these tests help in identifying cases promptly and accurately. This early diagnosis is crucial for initiating effective treatment and reducing the spread of the virus.
Additionally, the widespread availability of WHO PQ Listed Co-Branded Hepatitis C Rapid Tests supports various global health initiatives and programs aimed at combating Hepatitis C. The tests contribute to improving health outcomes and managing the disease more effectively, aligning with global goals to reduce Hepatitis C prevalence and impact.
Future Directions and Innovations
The future of Hepatitis C diagnostics is poised for significant advancements, particularly with the continued evolution of WHO PQ Listed Co-Branded Hepatitis C Rapid Tests. Ongoing innovations aim to improve test accuracy, reduce costs, and enhance ease of use. Emerging technologies, such as digital health solutions and point-of-care testing, are expected to play a crucial role in these developments.
Furthermore, collaborations between manufacturers, healthcare providers, and global health organizations will continue to drive progress. By focusing on improving test performance and expanding access, these innovations will contribute to more effective Hepatitis C control efforts and ultimately, better health outcomes for patients worldwide.
Conclusion
In summary, WHO PQ Listed Hepatitis C Rapid Tests represent a significant advancement in the fight against Hepatitis C. The rigorous standards of WHO PQ Listing ensure that these tests are of the highest quality, providing accurate and reliable results. The benefits of co-branding further enhance their credibility and distribution, making them essential tools for global health initiatives.
Jal Medical’s involvement in producing WHO PQ Listed Co-Branded Hepatitis C Rapid Tests underscores the importance of collaboration and innovation in improving diagnostic solutions. By leveraging their expertise and adhering to international standards, Jal Medical contributes to the broader efforts to manage and control Hepatitis C, ultimately supporting global health and enhancing patient care.

